Protective Coatings for Durable Concrete
M N Ramesh, Executive Director, Ecmas Construction Chemicals Pvt. Ltd
Introduction
Reinforced concrete is a composite material. Its structural performance is realised only when concrete and steel act in unison during the service life of the structure. The compressive and tensile loads are carried by concrete and steel respectively. Steel protects concrete from cracking under tensile loads and concrete protects steel from corrosion by providing an alkaline environment around it. So long as this happens, the reinforced concrete structures perform satisfactorily.
Even though the concrete can be a very strong material, it is also subjected to deterioration. Concrete can be porous so that chemicals can penetrate the pores and attack the paste. The paste and aggregate can also be worn down by physical impact and abrasion. Water can penetrate concrete, freeze and expand inside it when the temperature drops, and ultimately weaken the concrete from within. In addition, if the concrete has reinforcing steel bar (rebar) to impart additional strength and other properties, the rebar can corrode if moisture, oxygen and chloride ions penetrate the concrete. Corrosion or rebar contributes to the deterioration of concrete.
Various external hostile environmental substances, such as, water, carbon dioxide, oxygen, chlorides, sulphides and biological organisms are transported from the atmosphere into the concrete and attack steel and concrete in different mechanisms causing premature deterioration of reinforced concrete challenging its durability resulting in premature failure of the structures. The entry of the harmful agents can be restricted and or avoided by providing the barrier surface coatings on the concrete, thus conservation of structural integrity is achieved during its service life.
The Symbiosis
Failure Mechanism
Concrete is still the most durable material known to mankind. But it is also a fact that certain adverse environments affect the durability of concrete and reduce its service life. In an aggressive environment corrosion of reinforcing steel is one of the most important and prevalent deterioration processes. Concrete under normal exposure conditions protects reinforcing steel against corrosion. This protective behaviour of concrete is attributed to its high pH and the formation of a protective film on the surface of the embedded steel.
Reinforced concrete being a composite material relies on the high compressive strength of concrete and the high tensile strength of steel for its mechanical performance. Steel has poor corrosion resistance and concrete has good anti-corrosion properties. The hydration process of concrete leads to the formation of hydroxides which raises the pH level of the cement to around 12.5 and provides a stable oxide layer on the steel surface, which prevents the anodic dissolution of the steel.
Failure Mechanism
Reinforced concrete failure is caused by the corrosion of the steel reinforcing bars as a result of the destabilization of the oxide layer. When the passivity of the steel partly or completely breaks down, either as result of carbonation or chlorides, the corrosion will start. This means that the electrochemical potential of the steel locally becomes more negative and forms anodic areas, while the other portions of the steel which have the passive layer intact will act as catchment areas for oxygen and will form Cathodic areas. In spite of the development of high performance concrete from the early 1970s until today, it is evident that the application of high performance concrete in conjunction with measures such as, thick concrete cover and corrosion inhibitors are not necessarily good enough for ensuring high durability of concrete structures in the aggressive environments.
Deterioration Mechanism
There are various mechanisms of concrete deterioration. But the present discussion on the topic of protective coating focuses only on the factors influenced by the atmospheric exposure of reinforced concrete. Among them the very common ones are Carbonation, chloride attack and attack by sulphides.
Carbonation
Figure 1 : Corrosion of steel reinforcement in concrete
Reinforced concrete is a very strong building material and is basically concrete strengthened with steel bars. Reinforced concrete will begin to slowly corrode when exposed to the natural environment. Fresh concrete is highly alkaline because of hydration products such as calcium hydroxide. This environment protects the steel reinforcement bars from corrosion. However, carbon dioxide and moisture at the surface of the concrete can react with these products to produce calcium carbonate. Initially carbonation is restricted to a thin surface layer, but with time the carbon dioxide diffuses inward from the surface and the zone of carbonation extends gradually into the concrete.
The presence of pores and capillaries in the concrete facilitates the progress of the carbonation ‘front’. Good quality, dense concrete delays the development of carbonation and slows down the first stage of the corrosion process. Obviously the deeper the layer of concrete over the steel reinforcement the better protected it will be. This protective layer of concrete is called the ‘cover’.
Carbonation of the concrete does not in itself lead to a loss of structural adequacy, but when the zone of carbonation extends to the steel the protective action of the concrete is largely lost, primarily because the calcium carbonate has a reduced PH value. This then allows positive Fe+2 ions to form which join with dissolved oxygen in water to produce Fe2O3 (iron oxide or rust) and the process of corrosion of the reinforcing steel commences. The process of corrosion is greatly accelerated if chloride ions (from salt) are present. The chloride is not used in the chemical reaction to form rust but seems to assist in the formation of anodic and cathode regions in the metal. Figure 1 is the schematic representation of corrosion of steel in concrete.
Rate of Carbonation
Figure 2 : Corrosion of reinforcing steel due to carbonation
The rate of carbonation is strongly influenced by the concrete strength, permeability, relative humidity, depth of concrete cover, moist curing period and exposure condition. A good impermeable concrete having more concrete cover protects against the ingress of CO2. Due to formation of carbonic pore solution, there could be reduction of pH as low as 8.3 and consequently greater possibilities of enhanced reinforcement corrosion. When pH is less than 5 and the humidity is above 50%, an acid environment occurs which leads to extensive reinforcement corrosion. But when pH is in between 5.5 to 8.5 and humidity is above 50% at that time neutral environment reinforcement corrosion will begin which is moderate corrosion. But if the pH is over 8.5 and irrespective of any value of humidity an alkaline environment will occur where reinforcement is protected.
Relative humidity plays an important role for diffusion of CO2 through the concrete. Relative humidity lesser than 50% and higher than 80% does not affect much for CO2 diffusion since the pores in the concrete become either too dry or almost saturated respectively thereby not creating any vapour pressure for the process of diffusion. The critical moisture content for carbonation of concrete was found to be around 80%. Similarly the optimum climatic conditions for carbonation are relative humidity ranging between 50% and 70%, with wetting and drying cycles enhancing the reaction. Indian researchers have estimated the initiation of corrosion in coastal environments will take place nine years sooner considering the factors affecting for the carbonation such as CO2 concentration as 6%, the mean temperature as 30° C and the mean annual relative humidity as 74%.
Thus corrosion of reinforcement is an extreme complex electrochemical reaction in which iron combines with oxygen to form iron oxides and iron hydroxides which are rust products. These rust products expand five to eight times as a result occupy more volume than un-corroded steel causing internal stresses due to confinement of corroding steel. These internal stresses results in cracking of concrete cover. After this initiation phase of corrosion, those cracks become source of further ingress for deleterious substances for propagating into larger cracks, delamination and spalling of concrete as shown in Figure 2.
As the main cause of deterioration of concrete in the carbonation is due to permeation of concrete, a barrier coating applied on the surface of the concrete will prevent the diffusion of the harmful agents into the concrete and reaching reinforcing steel.
Chloride attack
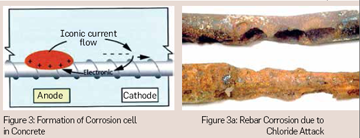
Chloride ions diffuse through the porous concrete cover at differential concentrations due to differential porosity conditions exists in the cover concrete. The diffused ions reside on the surface of the reinforcing steel at the varying concentrations at which they diffuse into the cover concrete. The regions of the reinforcing steel where the chloride concentration is higher become anode and adjacent area, where the lower concentration of the chloride ions exists, becomes cathode. The pore water or the absorbed water in the concrete will act as an electrolyte and a corrosion cell is formed resulting in pitting corrosion of reinforcing steel. Figures 3, 3a showing the formation of corrosion cell in concrete and chloride attack on Reinforcing bar. Surface coatings are used as barrier for the diffusion of water soluble chloride ion in the concrete thereby reducing the risk of corrosion of rebars.
Sulphate Attack
Sulphate Attack for Concrete
The deterioration of concrete due to sulphate attack is considered a complex problem and it depends on many parameters related to materials and exposure conditions such as: Parameters related to material properties, Parameters related to hydrated concrete properties like Pore structure, Permeability, Diffusivity, Mechanical properties etc and thirdly, Parameters related to exposure conditions like types of ions associated with Sulphate, Concentration of Sulphate, Time of exposure, Temperature of exposure etc.
 |
Sulphates or Sulphides from the atmosphere or ground water, come in contact with concrete and chemically react with C3A (Tri-calcium aluminate) to form an expansive compound known as ettringite (tri-calcium alumina-sulphate). This compound gets into all porosities, capillaries in the concrete matrix and expands 6 to 10 times creating an enormous internal busting pressure in the concrete and cracks. Although sulphate attack destroys concretes, it also paves way to the entry of CO2 and Cl- ions into the concrete causing a total destruction of concrete and reinforcing steel.
Protective Coatings
The protection of concrete should actually begin at the conceptual stage and meticulous strategies be adopted for protecting the concrete from both internal and external environments. Various coating materials and application methods for concrete surface repair and strengthening have been developed. However, selection criterion for these materials has not been established yet at the current moment. Selecting procedures of concrete coating materials must focus on deteriorating mechanisms diagnosed carefully by the conditions of target structures.
Components of Coatings
Figure 4 : Components of Coating
All organic coatings consist of three basic components:
- Solvent,
- Resin, and
- Pigment.
Not all coatings contain solvent and pigmented components. There are solvent-free (100 per cent solids) coatings and clear, pigment free coatings, but not resin -free coatings. Coating chemical formulators commonly group solvent, resin, and pigment components into two general categories. The first category combines the solvent and the resin together. The solvent portion is called the “volatile vehicle,” and the resin portion is called the “non-volatile vehicle.” The combination of the solvent and the resin, where the resin is dissolved in the solvent, is called the “vehicle.” The second category is the pigment. Pigments are additives that impart specific properties to the coating and are subdivided into two general categories: (1) colour and (2) inert and reinforced. Figure 3 illustrates the relationship of these components. When a coating is applied, the solvent evaporates during the curing process, leaving only the resin and the pigment components on the substrate. The remaining resin and pigments are sometimes called the “coating solids,” and they form the protective film for corrosion protection.
Solvent
Organic solvents are formulated into coatings to perform three essential functions:
- Dissolve the resin component
- Control evaporation for film formation; and
- Reduce the coating viscosity for ease of application.
Solvents will also affect dry film adhesion and durability coating proper ties. In general, resins that are less soluble will require either more solvents or stronger solvents to dissolve the resins. The terms “solvents” and “thinners” are often used interchangeably, but there are distinctions within and between the two terms.
The term “solvent” can imply two different usages: (1) the solvent or solvent blends in the coating formulation at predetermined concentration levels; or (2) cleaning solvents in strong concentration strength for cleaning brushes, rollers, hoses, and other equipment. The usage of the term “thinner” (a thinner is a solvent) is most often associated with the coating applicator adding a thinner to a coating container (normally about 475 ml thinner to 3.75 litres of coating ) to reduce the viscosity for ease of application . Adding thinner to a coating in the field is often called “field thinning.”
Resin
The resin (frequently called binder) is the film forming component of a coating. Resins are typically a high molecular weight solid polymer that forms large repeating molecules in the cured film. The primary purpose of the resin is to wet the pigment particles and bind the pigment particles together and to the substrate (hence, the term “binder”). The resin imparts most of the coating properties. The various types of resins formulated in a coating will display distinct properties. These properties are:
• Mechanism and time of curing
• Performance in service exposure type
• Performance on substrate type
• Compatibility with other coatings
• Flexibility and toughness
• Exterior weathering
• Adhesion
• Breathability (water vapour transmission ability)
No single resin can achieve a high degree of success in meeting the above coating properties with wide variations associated with each property. Therefore, generic coating types are generally classified by the primary resin type used in the coating formulation. Typical resins are acrylics, alkyds and epoxy polymers.
Pigment
Pigments are insoluble and are the heavier solid portion of a coating that typically settles to the bottom of the container. Pigments are additives to the coating formulation that impart specific properties to achieve the desired film properties. Pigments impart colour and aesthetics to the coating.
Coating Types
Coating types can be classified under three systems as under. Unlike coatings for steel substrates, protective coatings for concrete do not in most cases require or include inhibitive or sacrificial pigments to provide protection. Coatings applied to concrete are typically barrier coatings. They provide protection by becoming a physical barrier, or shield, isolating the concrete from its immediate environment. A barrier coating must prevent aggressive liquids and gasses from passing through it and reaching the concrete.
Barrier - A coating that forms a barrier between the concreter surface and prevents the entry of harmful agent into the concrete body by many of the transport mechanisms such as absorption, capillary suction, diffusion etc. they are essentially film forming agent on curing. Examples are acrylics, epoxies, coal tar epoxies etc.
One of the important properties of a barrier coating is the permeability. The permeability of a barrier coating's film depends on its moisture vapor transmission (MVT) rate. The MVT rate is determined by how fast water molecules pass through and move around the spaces between the resin molecules. The effectiveness of a coating in preventing permeation depends on how closely and tightly bound the molecules of the resin are to one another. The coating's effectiveness also depends on the type of resin molecule and the amount and type of pigment. Cross-linking is a measure of the degree of intense bonding of coating resins.
The lower the permeability of a barrier coating, the more protective the coating is. Basically, the higher the degree of the coating resin's cross-linkage, the lower the permeability, the better the adhesive bond of the coating to the surface, and the better the overall protective barrier. These intermolecular spaces between the resin molecules are much larger than the water molecules and should not be confused with physical holes (pinholes) in the coating film. Pinholes in the coating film are generally considered defects and should be repaired. Spaces between resin molecules are not defects.
The barrier properties of coatings can be improved by adding reinforcement fillers to the resin. Fillers come in a variety of forms, such as silicate aggregates (sand), glass or mica flakes, fibers, ad woven fiberglass ( incorporated as a mat in the resin system as it cures). The addition of fillers physically increases the length of the path that the intruding liquid or gas molecules must take to penetrate the coating. Flake materials form layers of overlapping platelets, parallel to the concrete surface, somewhat like shingles on a roof. Fillers and fiberglass mat can also be added to improve the barrier coating's physical properties, such as impact and abrasion resistance.
Inhibitive - A penetrant or a primer that is slightly soluble in water or a solvent that forms a chemical inhibitor and effectively coats the walls of the capillaries in concrete. They generally impart properties like hydrophobicity for the concrete but allow the water vapour transmission through them. Examples are Silane - siloxanes etc.
Galvanic. - Zinc-rich primer coatings that provide galvanic or Cathodic protection to ferrous metal (zinc sacrifices itself to protect the ferrous metal). Galvanic coatings are effective only if applied directly to bare metal. They prevent formation of incipient anodes in the reinforcing steel in a typical patch repair situation.
Generic Coatings
Acrylics coating for concrete
The following generic coatings and general descriptions are typically specified by the consultants.
Acrylics - In water-borne acrylic coatings, the resins are dispersed in water to form a water emulsion. Water –borne acrylics are specified for atmospheric exposures as a primer or top coat and have excellent colour and gloss retention. Acrylics cure by coalescence. They are breathable and UV stable. Good barrier for carbon dioxide.
Alkyds - Alkyd s are normally natural oils (soya, Tung, styrene) that have been chemically modified to improve cure rate, chemical resistance, and hardness. Phenolic modified alkyds are specified as a primer, and silicone alkyds are specified as the topcoat for atmospheric service exposures primer, as well as the topcoat for atmospheric service exposures especially for metals. They are not suitable for alkaline (concrete or masonry) surfaces or environments. Alkyds cure by air oxidation of drying oils.
Bituminous - Bituminous coatings are heavy-bodied materials applied with a cutback solvent. They have good moisture barrier resistance and fair to good chemical resistance but are not resistant to solvents. Commercial bituminous products are specified on a limited basis by consultants for protection of aluminium surfaces in contact with cementitious material or steel and copper cable weld connections. Bituminous coatings cure by solvent evaporation.
Bituminous Coatings for concrete
Epoxy, Amine. - Amine epoxies are two-component coatings that are catalysed (hardened) by an amine curing agent to produce a hard, tightly bonded, chemical resistant (alkali, acid, and solvent) product, but they are moisture and temperature sensitive during application. They are specified for burial and immersion service exposures, but they will fade and chalk in direct sun light. Amine epoxies cure by chemical reaction.
Epoxy Amine Coatings for Concrete
Epoxy, Polyamide. - Polyamide epoxies are two component coatings that are catalysed by a polyamide curing agent to produce superior resistance to water and salt solutions, but they do not provide the chemical resistance of the amine epoxy. Polyamides have a greater flexibility than the amine epoxies. They are specified for burial and immersion service exposures, but they will fade and chalk in direct sun light. Polyamide epoxies cure by chemical reaction.
Polyurethane Coatings for Concrete
Epoxy, Coal Tar. - Coal tar epoxies are generally an amine or polyamide epoxy modified with coal tar pitch resin to produce a high-build film that has good chemical resistance and excellent water resistance. They have a tendency to become brittle with age and delaminate between coats or beneath repair patches. They are specified for burial and immersion service exposures, but they will fade and chalk in direct sunlight. Coal tar epoxies cure by chemical reaction.
Epoxy, Fusion-Bonded. - Fusion bonded epoxies (commonly called powder coatings) are complete coatings in powder form. There are two application methods, fluidized-bed and electrostatic. In the fluidized-bed method, the metal items are preheated to a fusion temperature and immersed in the powder-epoxy solution. In the electrostatic method, the epoxy powder particles are charged with high voltage, and the metal item is then sprayed. After spraying, the item is placed in an oven to cure at about 180 to 350°C. Fusion bonded epoxies are specified for reinforcing steel, but they become brittle and fail to protect steel in the long run. They act as barrier to the steel getting direct contact with the alkaline concrete and deprive the reinforcing steel to have natural protection by the alkaline concrete.
Inorganic Zinc Primers - Inorganic zincs are primers that incorporate a high loading (Kg per litre ) of metallic zinc for pigmentation (hence, the term “zinc-rich”) and are either solvent or water based . Depending on the solvent and resins used, the coating may be a zinc-rich epoxy or urethane. These coatings are exclusively primers because they provide galvanic or cathodic protection to steel substrate. Inorganic zincs are specified for atmospheric and immersion service exposures, but they can be top coated to extend their service life. Suitable topcoat material selection is required to prevent out-gassing from the inorganic zinc that produces small pinholes in the top coat. Suitable for steel bars and structural steel section buried in concrete contaminated with chlorides.
Polyurethane - Technically, polyurethane is a subclass of urethane. Two-component polyurethane is created by chemically combining a polyisocyanate and a polyol to produce an isocyanate that has a two mode cure mechanism of solvent evaporation and chemical reaction. Generally polyurethanes are specified for top coating compatible (i.e. same manufacturer) amine and poly amide epoxies to protect against direct sun light or U V and to provide specific colours. Polyurethanes are specified for atmospheric and partial or fluctuating immersion service exposures.
Urethane - Urethane coatings vary widely in formulations for specific service environments and application requirements .Many times single-component; moisture-cured urethanes are specified. They cure from moisture in the atmosphere and can be applied to damp surfaces that do not have free moisture present. These urethanes are formulated with various pigmentations and are specified in several combinations to suit the intended service exposure. These urethanes are specified for atmospheric, burial, and immersion exposures.
Coatings that Breathe
As discussed earlier, the curing of new concrete often results in the release of substantial quantities of water. If this water is trapped between the coating and the concrete, it can cause the coating to lose adhesion or form blisters. It is sometimes necessary, therefore, to use coatings that "breathe." These coatings allow water vapour (the gas form of liquid water) to pass through them. However, care should be taken when selecting a more permeable coating to ensure that the service conditions are not beyond the range of the coating. The higher the permeability, the lower is the resistance in preventing water or other chemicals from the outside environment from passing through the coating. It is the coating manufacturer's and the specifiers responsibility to select and furnish the coating with the right degree of " breathability" and "permeability rating" for the intended service (use) of the coated concrete.
Functional requirements
The coatings have two main functions viz. providing protection against harmful agents thereby increasing the durability and providing aesthetic appearance to the structure. To achieve these functions, the coatings should have the following attributes: Table.1 shows the properties of different resins available in the market.
- Good adhesion to the surface to be coated
- Resistant to alkalis, as the coatings are applied on alkaline concrete
- Resistance against CO2, sulphate and Chlorides to provide barrier property
- Good flexibility as the structural members undergo dimensional variation due to cyclic loads
- Excellent weathering resistance
- Breathability- should allow water vapour transmission through the coating to avoid blistering of the coatings; a durability requirement.
- Resistance for UV exposure- a durability requirement
- Low susceptibility to staining
- Good resistant against growth of fungi, algae moss etc.
Table 1 Comparison of performance of various resin systems
However, It should be noted that all resin materials are not totally resistant and impermeable to all aggressive agents and do not provide a total protection. Chemical/physical degradation of resins and de bonding of coatings are the major phenomena affecting the durability of surface protection. The mechanisms of destructive processes in such heterogeneous materials as resin composites are complicated and not completely understood. Degradation of resins mainly involves swelling, dissolution and scission of molecular chain bonds.
A wide variety of reactions is possible for resin degradation. The transport of gases and liquids aggressive to substrate into or through the coating is the major problem of its delamination. Various mechanisms of deterioration of resin composites and coatings are summarised in Table 2. There are many parameters that influence the deterioration process of coatings, such as chemical agents, temperature, solar radiation, pressure, abrasion, cyclic temperature-moisture changes etc.
All these parameters can occur simultaneously or they can be complementary to one another. The barrier may be subjected to continuous exposure or intermittent contact occasioned by splash, spray, or accidental wetting with aggressive substances.
Usually chemical/physical degradation and de-bonding of coatings is the major problem of deterioration leading to their cracking and delamination. On the basis of the degradation processes the basic requirements for protective coatings of concrete structures in aggressive environments can be formulated. They are as follows:
- Resistivity to chemical/physical actions
- Low permeability to water, solutions and gases
- Good bond to concrete
- Sufficient flexibility to avoid cracking caused by thermal or mechanical movements
- Similar physical properties of the overlay material and underlying concrete
- Adequate abrasion or skid resistance.
- Resistivity to chemical action of concrete and humidity in concrete.
- Bridging of fine cracks in concrete
Principles of protective coatings design
The design of an appropriate protective system for new or existing structures is a complex process involving:
- Identification of service environment of the particular structure in the original design
- Identification and assessment of the condition state and deterioration (if any) of the existing structure
- Selection of the appropriate protection system
- Definition of coating parameters: type of binder, formulation, covers thickness
- Anticipated time between periodic recoating.
- The performance/efficiency of any coating depends on its chemical/ physical resistance to disintegration, permeability, extensibility, mechanical resistance (example- abrasion, punching), and adhesion to concrete.
Table 2: Typical causes of failure of coating systems for concrete
Traditionally codes and recommendations contain requirements for structural design in terms of resistance – load format. Similarly to that design concept, the design of coatings must be developed on the basis of deterministic or probabilistic analysis taking into account the aggressive environment as an action and coating performance as resistance. In particular, aggressive actions as well as material and geometric properties of coatings may vary substantially. Limit state functions can be represented in resistance or lifetime format:
g(t) = R(t) - S(t) = R0?R (t)θR - S(t)θS ≥ 0,
g(t) = tθt - td ≥ 0, for all 0 < t ≤ td
Where g(t) is the margin of safety with g(t) > 0 denotes safe and g(t) ≤ 0 denotes failure; R0 is protective barrier capacity in the un-degraded (original) state; ?R(t) – degradation function; θ − uncertainty of the calculation models and errors in data observation and recording; t – the time of assessment; td − the design or target service life.
Once the limit state functions have been developed, the reliability of coating can be evaluated. The reliability verification of coating in relation to a given mode of failure in a given period of time may be defined as:
P{t} = P{g(t) ≥ 0} = P{R0?R (t)θR ≥ S(t)θS } ≥ Pt arg ,for all 0 < t ≤ td, (29)
Where, Pt arg is an acceptable level of structural reliability.
The service life of the coating is defined when the reliability falls below an acceptable level.
Different resins respond in a different way to the influence of aggressive environments. The time dependent monotone decreasing degradation function ?R(t) can be expressed in different forms (linear, parabolic, quadratic, etc.) with the following boundary conditions.
at t = t0, ?R(t0) = 1,0,
at t = td, ?R(td) = ?min.
The design of resin coatings requires checking of their performance in general using the following four conditions:
- The condition determining the chemical/physical resistance with R(t) = R0φR(c0;t) and S(t) = Rmin, where, R0 and Rmin are initial and minimum acceptable resistance of coating, respectively; φR(c0, t) – degradation function of coating in a given exposure c0 after time t;
- The condition determining the penetration through the coating with R(t) = ccr and S(t) = c(dpc,t), where ccr and c(dpc;t) are the critical and expected concentration of aggressive substances, respectively, on the surface of the concrete;
- The condition determining the cracking of the coating with R(t) = fpt(t) [εpt(t)] and S(t) = σmax (εmax), where fpt(t) [εpt(t)] is tensile strength (strain) of resin and σmax (εmax) is maximum stress (strain) in coating;
- The condition determining the delamination (separation) of the coating with R(t) = τcon [KIc(t)] and S(t) = τmax [KIcor(t)] or R(t) = Dcr and S(t) = D(t), where KIc(t) and KIcor(t) are critical initial and after exposure in aggressive environment stress intensity factor, respectively; Dcr and D(t) is critical and expected degree (area or %) of delamination, respectively.
Surface Preparation
Surface preparation also plays an important role in enhancing the service life of a coating in addition to the selection of suitable kind of coating material. Inadequate surface preparation eventually results in premature failing of a coating system. Thus, cleanliness of the substrate and removal of surface contaminants become an essential and integral component of a coating system application. Following are the most common types of surface contaminants and the consequences of not removing them:
Rust - Rust is the corrosion by product of steel and may be loose or may adhere relatively tightly to the substrate. Rust is porous and may include moisture, oxygen, and soluble salts. Rust will expand up to eight times the volume of the base metal consumed and further corrode the steel substrate, thus dislodging any coating applied over it.
Mill scale - Mill Scale is a heavy oxide layer formed during heat treatment of metals and is bluish in colour. Mil scale will eventually break loose from the steel substrate, taking the coating with it. Steel is anodic to mill scale (steel has a lower electrical chemical potential difference than mill scale); therefore, steel will corrode (sacrifice itself) to protect the mill scale.
Grease and oil - Grease and oil prevent a coating from adhering to the substrate.
Dirt and Dust - prevent proper adhesion of the coating system.
Soluble Salts - Soluble salts deposited on a surface can remain on the surface, even after abrasive cleaning. Soluble salts will increase moisture permeation through the coating (osmotic blistering) and can accelerate the corrosion rate under the coating film (under film corrosion or under cutting). The most common soluble salts encountered in the coating industry are chlorides, sulphates, and metallic salts. The chloride ion is the most aggressive.
Water. - Water will prevent adhesion and may either produce flash rusting before the application of coating or may accelerate under film corrosion after coating application. Moisture in the liquid or frozen state will prevent adhesion of coating to the substrate and can disrupt curing reactions of coatings. Moisture contamination can cause several types of failure.
Chalk - Chalk is the residue left after the deterioration of the coating’s organic binder. Chalk results from exposure of the coating to direct sunlight or artificial UV light. All coatings chalk to some degree, but epoxies are more prone to chalk. Over coating chalked surfaces will result in poor adhesion and may result in delamination (separation of one coating layer from another coating layer) failure.
Deteriorated coatings - Old, loose, deteriorated coatings that are over coated may peel, delaminate , or lift from the substrate and take the new coating with them .
Concluding Remarks
- Concrete is a porous material having high gas, vapour and liquid permeability leading to deterioration of reinforced concrete structures. One of the ways to protect RC structures from corrosion is to use protective coatings. Frequently, the coating is the main option to protect the concrete structures in service. Many coating materials are not totally resistant and impermeable to all aggressive agents. It is necessary to well understand the mechanism of degradation of coatings materials to enable design of coatings with required barrier properties.
- The degradation of polymers is a complex interaction of physical and chemical processes leading to breakdown of its chemical structure as well as cracking and debonding of protective coatings. Classification of coating degradation has been done based on the nature of action.
- The mechanisms of degradation of coatings caused by aggressive actions are well understood and predictive models for the deterioration over time have been developed which can be applied to design of surface polymer coatings, to preserve concrete structures against deterioration.
- Design of coatings is based on deterministic or probabilistic analysis in the resistance – load format aggressive environment as an action and coating performance as resistance. Such a design of protective coatings is available in a simple form for engineering design purposes.
- There are a large number of materials and systems in the market that claim a variety of properties. Experience shows that the processes of coating deterioration and the loss of protection ability are very complicated. The physical and chemical reactions for each protective system in particular environments have to be determined experimentally.
Acknowledgements
This paper being a review paper is not based on original research. The author has compiled information from various source material and industry technical literature besides his own experience during his long term association in construction chemicals Industry and Remedial Engineering. The material compiled from various references is duly acknowledged.
References
- Suresh Chandra Pattanaik, Dr. Bitayanjay Das, ”Anti-Carbonation Coatings in Extreme Environment for Durable RC Structures”- Paper published in the proceedings of 2nd International Conference NUiCONE 2011at Institute of Technology, Nirma University, Ahmedabad from December 8-10, 2011
- A. Schweitzer, “Corrosion and Corrosion protection handbook”,
- C.G. Munger(NACE),” Corrosion Prevention by Protective Coatings”
- S.A. Bradford, “Corrosion Control “
- Tom N. Bortakm “Guide to Protective Coatings: Inspection and Maintenance”
- AP/M permaform “Protective coating for concrete structures cor+gard “